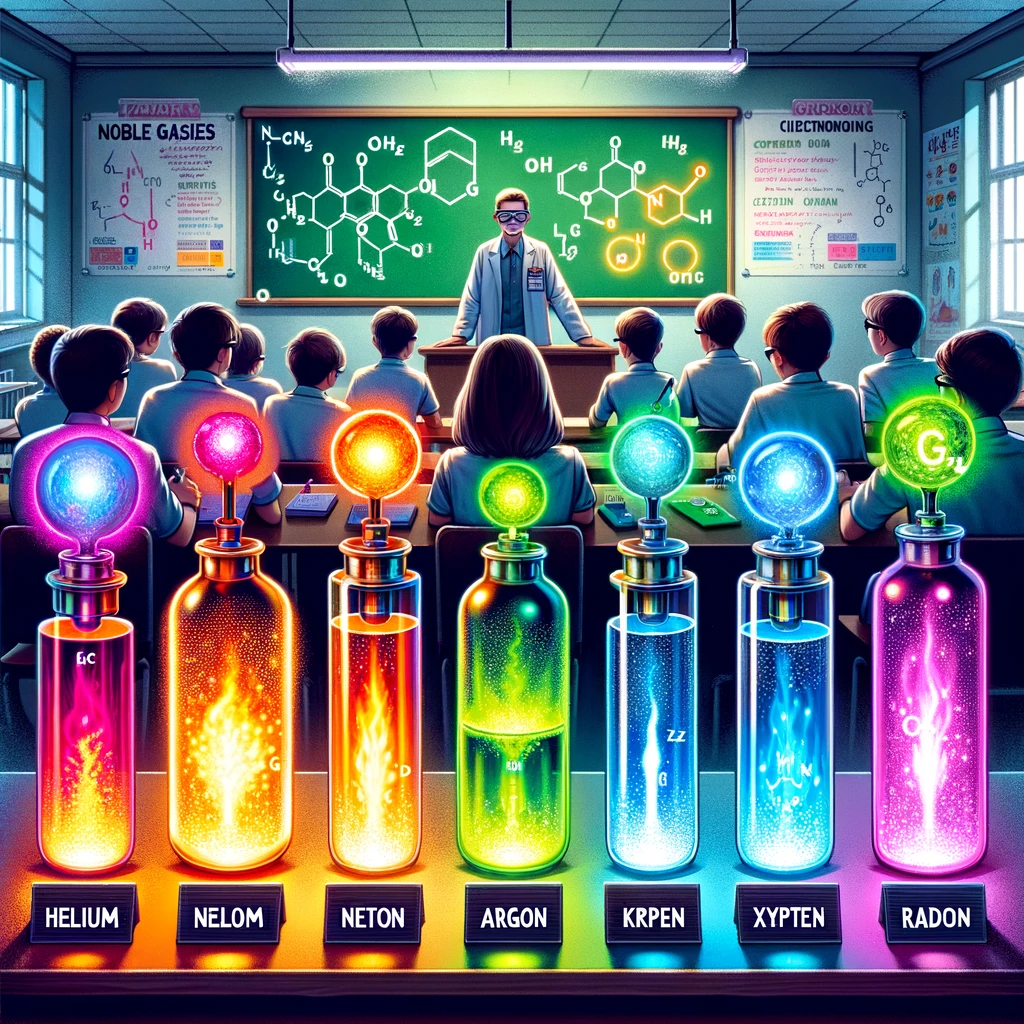
Noble gases, sometimes called inert gases, hold a unique place in the periodic table. These gases, including helium (He), neon (Ne), argon (Ar), krypton (Kr), xenon (Xe), and radon (Rn), are known for their lack of color, odor, and taste. Noble gases are nonflammable, making them incredibly stable under standard conditions.
Historically, noble gases were placed in Group 0 due to their full valence electron shells, contributing to their lack of reactivity with other elements. However, this group is now recognized as Group 18, reflecting a more accurate understanding of their properties and interactions.
Noble Gases: Key Takeaways
In a hurry? Don’t worry. Our critical takeaways on noble gases will give you a quick and easy summary of the main points:
🟠 Noble gases, including helium and argon, are unique elements known for being colorless, odorless, and chemically inert.
🟠 The distinctive inertness of noble gases, such as neon and xenon, stems from their complete outer electron shells.
🟠 Noble gases are vital for lighting, insulation, and healthcare, valued for their inertness and non-toxicity.
If you find noble gases challenging, don’t worry! Personalized tutoring or interactive chemistry lessons make these concepts more straightforward. Explore more chemistry topics and broaden your knowledge with our free World of Chemistry blogs.
What Are Noble Gases?
Noble gases are the final column on the right of the periodic table. They are characterized by their electronic configurations, which result in very low reactivity. This distinct group comprises six naturally occurring gases: helium, neon, argon, krypton, xenon, and radon. Each element has unique applications and occurrences in the natural world, from filling balloons to illuminating neon signs.
Defining Characteristics of Noble Gases
The defining feature of noble gases is their complete outer electron shells, which make them extraordinarily stable and inert. This electronic configuration prevents them from readily forming compounds with other elements. The term “noble” reflects their resistance to participation in chemical reactions, similar to noble metals like gold and platinum that resist oxidation and corrosion.
The Role of Noble Gases in the Periodic Table
Noble gases, located in Group 18, are pivotal for understanding the periodic trends and electronic configurations of elements. Their full outer shells are a benchmark for the stability other elements strive to achieve through chemical bonding. Initially thought incapable of forming compounds, research has revealed that heavier noble gases like xenon and krypton can engage in chemical reactions under specific conditions, challenging and expanding our understanding of chemical bonds.
Are noble gases tricky for you? A chemistry tutor can provide personalized lessons tailored to your needs, making organic and inorganic chemistry understandable and enjoyable.
Deep Dive into the Properties of Noble Gases
Noble gases are renowned for their exceptional stability and low reactivity, which set them apart from other elements in the periodic table. This section explores the scientific basis behind these properties and what makes noble gases unique.
The Science Behind Inert Gases
The inertness of noble gases stems from their electronic configurations. Each noble gas has a complete set of electrons in its outer shell, with helium having two electrons and the rest having eight. This full valence shell is energetically favorable and renders the noble gases chemically stable, meaning they have little tendency to lose, gain, or share electrons with other atoms.
This stability is a key reason why noble gases do not readily form compounds under normal conditions. Their high ionization energies and negligible electronegativities further contribute to their nonreactive nature. Ionization energy is the energy required to remove an electron from an atom. This energy is significantly high for noble gases, indicating that stripping electrons away from them is challenging.
Fundamental Properties That Make Noble Gases Unique
- Nonreactivity: The most distinguishing property of noble gases is their lack of chemical reactivity. This nonreactivity makes them valuable in applications that require an inert atmosphere, such as protecting reactive metals during welding or preventing oxidation in scientific experiments.
- Monatomic Gases: Unlike most elements, noble gases exist as single atoms rather than molecules under standard conditions. This monatomic form is another consequence of their full outer electron shells, which negate the need for bonding with other atoms to achieve stability.
- Low Boiling and Melting Points: Noble gases have some of the elements’ lowest boiling and melting points. This characteristic is due to their weak van der Waals forces, the only type of intermolecular forces present, which are insufficient to hold the atoms together in a liquid or solid form at higher temperatures.
- Density: The density of noble gases increases with their atomic number. However, all remain less dense than air, with helium and neon significantly lighter. We exploit this property in airships and balloons, where lifting capability is essential.
- Colorless, Odorless, and Tasteless: In their natural state, noble gases are colorless, odorless, and tasteless. This makes them indistinguishable from human senses and ideal for use in environments where chemical contamination must be avoided.
Anyone curious about chemistry in daily life can explore simple experiments or consult a chemistry tutor to discover more about the science behind these everyday phenomena.
Exploring Each Noble Gas
Noble gases have a distinct place in nature and technology thanks to their unique properties. This section delves into the specifics of each noble gas, examining its applications, uses, and groundbreaking discoveries that have broadened our understanding of its chemical behavior.
Helium (He): More Than Just Balloons
Helium in Medicine and Technology
Helium, the second lightest and most abundant element in the observable universe, plays critical roles beyond filling balloons. In medicine, helium is used in respiratory treatments for conditions like emphysema and asthma due to its low density, facilitating easier breathing. Its low boiling point in technology makes it invaluable in cryogenics, particularly in cooling superconducting magnets in MRI scanners and particle accelerators.
The Lift Factor: Helium in Balloons and Airships
The most recognized use of helium is in balloons and airships, where its low density and nonflammability offer a safe alternative to hydrogen. Despite its widespread association with party balloons, helium’s application in scientific research balloons and airships underscores its value in atmospheric studies and transportation.
Neon (Ne): Lighting Up Our World
The Magic Behind Neon Signs
When electrified, neon gas emits a bright reddish-orange glow, which has become synonymous with neon lighting used in advertising signs. The vibrant colors produced by neon and its inertness, which prevents it from reacting with other substances in the lamp, have made it a staple in visual displays and art installations.
Crafting Colors with Neon
While pure neon produces a reddish-orange light, mixing neon with other gases or using different coatings inside the lamp tubes can create a spectrum of colors. This versatility is why neon lighting is popular in commercial signage and artistic expressions, offering durability and energy efficiency.
Argon (Ar): The Invisible Protector
Argon’s Role in Welding and Preservation
Argon gas is utilized extensively in welding and metal fabrication, acting as a shield gas to protect the weld area from atmospheric gases that might contaminate the weld. Its inertness ensures that it does not react with the metal, leading to cleaner and stronger welds. Argon also preserves historical documents and materials, providing a nonreactive atmosphere that helps prevent decay and degradation.
Enhancing Windows with Argon Gas
In double-glazed windows, argon fills the space between panes, improving thermal insulation due to its low thermal conductivity. This application highlights argon’s role in energy efficiency, reducing building heating and cooling costs.
Krypton (Kr): Beyond Superman’s Home
Illuminating the World with Krypton
Krypton’s use in lighting extends from high-intensity discharge lamps utilized in airports and roadways to more efficient light bulbs. Its ability to produce a bright white light makes it suitable for high visibility and energy efficiency applications.
Krypton’s Contribution to Energy Efficiency
Like argon, we use krypton to insulate double-glazed windows, but its superior insulating properties allow for even thinner spaces between the panes. This application is precious in energy-efficient architectural designs, where maximizing insulation without compromising window size or clarity is essential.
Xenon (Xe): Lighting and Beyond
Xenon in Automotive and Cinema
Xenon, one of the heavier noble gases, has fascinating uses in lighting. In automotive headlights, xenon gas discharges produce a bright, white light that closely mimics natural daylight, enhancing driver visibility. This technology improves safety and adds an aesthetic appeal to modern vehicles. In cinemas, xenon arc lamps are powerful projectors, offering brilliant illumination that brings movies to life on the big screen.
Xenon’s Role in Medical Imaging
In medical imaging, xenon plays a pivotal role due to its unique properties. When inhaled, xenon gas acts as a contrast agent in lung imaging, allowing doctors to observe lung function in real time. This application is crucial in diagnosing and monitoring respiratory conditions, showcasing xenon’s invaluable contribution to healthcare.
Radon (Rn): A Noble Gas with a Dark Side
Understanding Radon Risks
Radon, unlike its noble gas counterparts, presents a health risk due to its radioactive nature. It is a decay product of uranium and can accumulate in homes, particularly in basements and lower levels, posing a risk of lung cancer upon prolonged exposure. The awareness and detection of radon in living spaces are critical for health and safety, emphasizing the need for regular monitoring and mitigation measures in affected areas.
Detecting and Mitigating Radon
Detecting radon gas is crucial in protecting yourself from its harmful effects. Radon test kits are available for homeowners to measure levels in their homes. If high levels are detected, mitigation strategies, such as improving ventilation or sealing floors and walls, can significantly reduce radon concentrations. These actions underscore the importance of proactively dealing with radon and ensuring a safe environment for you and your family.
The Impact of Noble Gases on Daily Life
Noble gases play a crucial role in many applications that affect our daily routines and the advancement of technology. Their unique characteristics, such as colorless, odorless, and chemically inert, make them invaluable across various fields.
Everyday Uses of Noble Gases
- Helium: Fills balloons; cools MRI magnets.
- Argon: Insulates double-glazed windows; protects welds.
- Neon: Lights up signs and decorations.
- Xenon: Powers cinema projectors.
- Krypton: Enhances energy efficiency in windows.
- Radon: Monitored for safety due to radioactivity.
Safety Measures for Noble Gases
Due to their unique properties, handling noble gases requires specific safety measures. Although noble gases are non-toxic and non-flammable, their inertness can pose risks in confined spaces. Proper ventilation is crucial to prevent asphyxiation, as noble gases can displace oxygen. For radon, which is radioactive, detecting and mitigating its presence in buildings is essential to reduce health risks. Using radon detection kits and improving home ventilation can significantly lower exposure levels.
Concluding Thoughts on Noble Gases
Noble gases, including helium, neon, argon, krypton, xenon, and radon, are crucial in everything from healthcare to technology. They have unique characteristics, such as colorless, odorless, and inert, making them indispensable.
For those eager to learn more, chemistry tutoring and lessons can offer deeper insights. Engaging with tutors or private teachers can provide a tailored learning experience, enhancing our understanding of noble gases and their role in our world.
Suppose you’re looking for a chemistry tutor. In that case, a simple search like “organic chemistry tutor Liverpool” or “inorganic chemistry teacher Edinburgh” on platforms like meet’n’learn can help you find the right private teacher for your needs.
Those who prefer group learning environments can easily find chemistry classes nearby by searching for “chemistry classes Leeds” or “chemistry lessons London” online, leading to local schools or educational centers.
Noble Gases: Frequently Asked Questions
1. What are noble gases?
Noble gases are inert, colorless, and odorless elements in Group 18 of the periodic table, including helium, neon, argon, krypton, xenon, and radon.
2. Why are noble gases inert?
Noble gases are inert because they have a full valence shell of electrons, making them stable and unreactive.
3. Can noble gases form compounds?
Despite their general inertness, heavier noble gases like xenon and krypton can form compounds under specific conditions.
4. Are noble gases dangerous?
Generally, noble gases are safe, but radon is radioactive and poses health risks, and all noble gases can cause asphyxiation in high concentrations without adequate ventilation.
5. How are noble gases used in everyday life?
Noble gases are used in lighting (neon signs), cooling (helium in MRI machines), and insulation (argon in double-glazed windows).
6. Where do noble gases come from?
Noble gases are extracted from the air through liquefaction and fractional distillation, except helium, which we obtain mainly from natural gas reserves.
7. Can noble gases conduct electricity?
As seen in neon lights, noble gases do not conduct electricity but can glow when electrified.
8. Is helium the lightest noble gas?
Yes, helium is the lightest noble gas and the second lightest and most abundant element in the observable universe.
References:
1. Britannica
2. Libre Texts Chemistry
3. Wikipedia